🌍✨ World Cancer Research Day in 3D - Marianna Kruithof-de Julio! ✨🌍
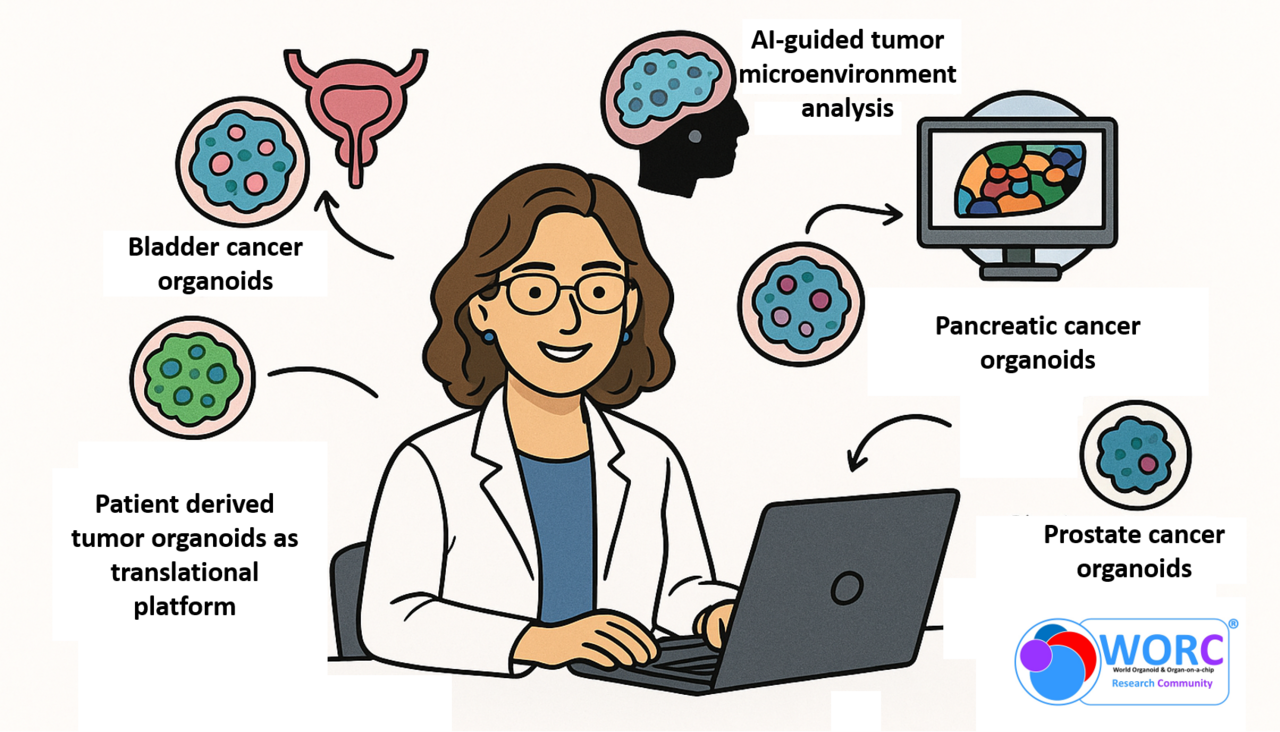
Prof. Kruithof‑de Julio works with a remarkable range of patient-derived tumor organoids, among them bladder, prostate, and pancreas. She uses them to study tumour heterogeneity, therapy response, and the tumour microenvironment. She combines organoid biology with multi-omics, AI, and innovative functional assays to advance precision oncology.
Here’s a snapshot of her recent work:
In her recent publication, “CRIPTO’s multifaceted role in driving aggressive prostate cancer unveiled by in vivo, organoid, and patient data” (https://doi.org/10.1038/s41388-024-03230-x), the authors investigated the role of CRIPTO (TDGF1) in prostate cancer progression. Using genetically engineered mouse models targeting Nkx3.1-expressing cells, the authors showed that CRIPTO depletion reduces tumor invasiveness, particularly at advanced stages, while CRIPTO overexpression alters organoid morphology and enhances tumorigenicity. Transcriptomic analysis revealed a CRIPTO/MYC co-activation signature linked to prostate-specific antigen (PSA) progression in human prostate cancer. These findings established CRIPTO as a driver of invasiveness and a potential biomarker and therapeutic target.
In the study “Overcoming limitations in current measures of drug response may enable AI-driven precision oncology” (https://doi.org/10.1038/s41698-024-00583-0), Kruithof-de Julio, Rapsomaniki, and colleagues highlighted major limitations in current drug response metrics, which impede the development of pan-cancer ML models for precision oncology. The study shows that many models fail to make truly personalized predictions, as they do not rely on molecular features. While z-scored IC₅₀/AUC values can improve prediction by comparing responses relative to the average patient, but this remains technically challenging. By contrast, even simple models perform well when trained in a drug- and disease-specific setting using omics data. Future progress will require better response metrics and the integration of organoid models, which hold strong promise for guiding personalized cancer therapies.
In the publication entitled “Bladder cancer organoids as a functional system to model different disease stages and therapy response” (https://doi.org/10.1038/s41467-023-37696-2), Kruithof-de Julio and her team established bladder cancer patient-derived organoids (PDOs) from multiple tumor stages and grades, which preserve histological, molecular, and multiclonal heterogeneity. Drug screening with standard therapies and FDA-approved compounds enabled the identification of candidate markers of treatment response, highlighting PDOs as a platform for precision oncology.
In her collaborative work with Gerald Schwank, “Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label therapy” (https://doi.org/10.1016/j.xgen.2022.100095 ), the authors established a pancreatic cancer organoid biobank of 31 genetically diverse lines, capturing the heterogeneity of primary tumors. Using CRISPR-Cas9 and drug screening, they identify drug–gene interactions, showing ARID1A missense mutations confer sensitivity to dasatinib and VE-821. An automated screen of 1,172 FDA-approved compounds reveals 26 hits, with in vitro and in vivo validation of emetine and ouabain. This study highlights tumor organoid biobanks as platforms for precision oncology and drug repurposing.
With so many organoid systems at her fingertips, Prof. Kruithof‑de Julio is redefining how we model cancer in the lab, capturing the complexity of human tumours and opening the door to truly personalized therapies.
✨In her talk on the 30th of September, Prof. Marianna Kruithof de Julio will be introducing her recent work freshly accepted in the Journal of Experimental & Clinical Cancer Research, do not miss it!✨


Please sign in or register for FREE
If you are a registered user on WORC.Community, please sign in