WORD+25 Xmas Meet-Up: Dr Korcsmáros and Dr Badder Introduction!
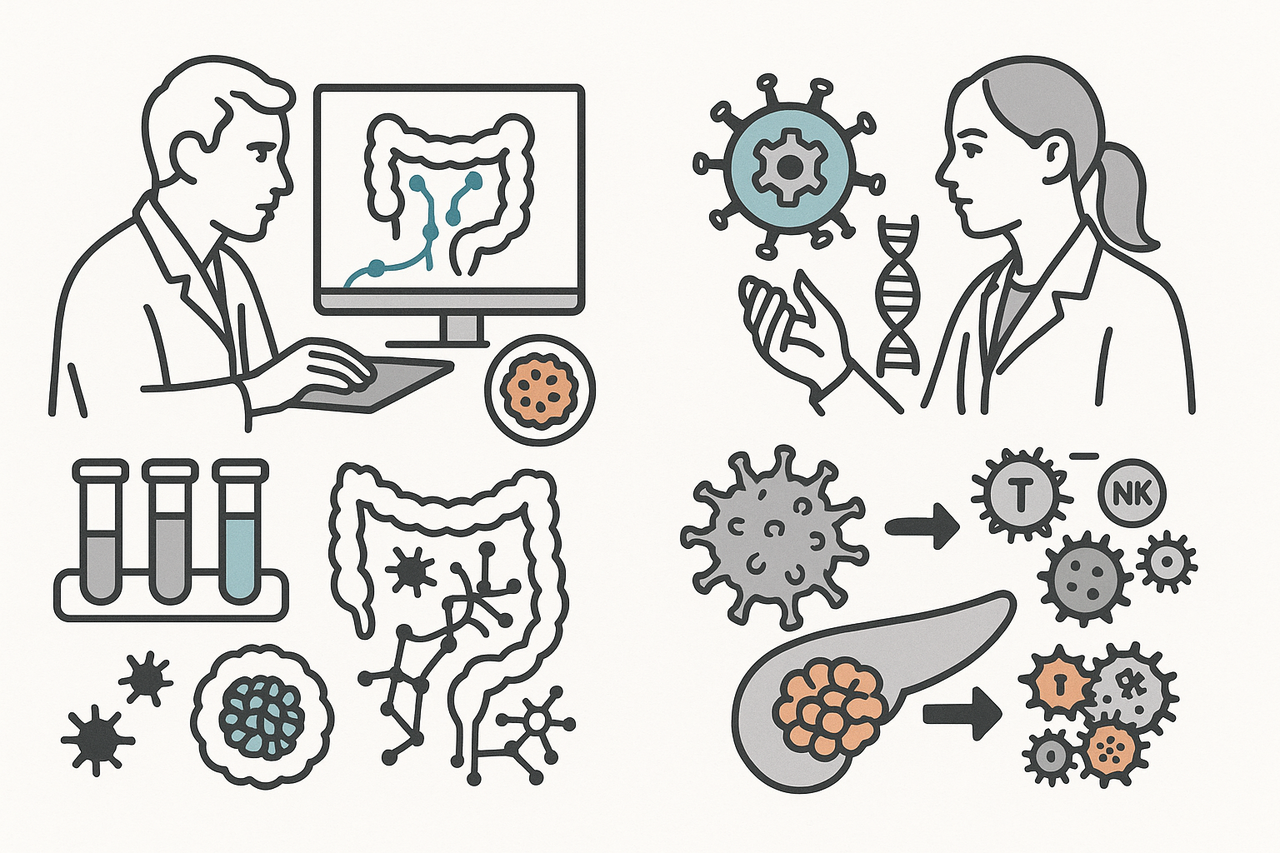
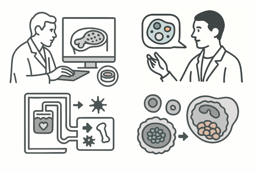
As the 4th of December event (WORD+25 Xmas Meet-Up) is only one month away, we would like to start introducing two leading voices in 3D biology, whose work spans organoids, cytokine-mediated interactions, and precision virotherapy.
Dr Tamás Korcsmáros (Imperial College London) leads a computational and experimental systems biology lab exploring how intestinal epithelia respond to cytokine signals, microbes, and cellular stress. In his work “Cytokine responsive networks in human colonic epithelial organoids unveil a molecular classification of inflammatory bowel disease” (https://doi.org/10.1016/j.celrep.2022.111439), Korcsmáros, Nick Powell, and colleagues employed human colonic organoids treated with cytokine panels to map dynamic signalling networks and uncover epithelial regulators involved in inflammatory bowel disease (IBD). In his review “Organoid-based Models to Study the Role of Host-microbiota Interactions in IBD” (https://doi.org/10.1038/s41416-024-02869-3), Korcsmáros, Marc Ferrante, and co-authors discuss how altered gut microbiota is central to IBD, highlighting that advanced organoid-based microfluidic models now enable ex vivo study of cytokine-epithelial-immune interactions, offering insights into disease mechanisms and paving the way for personalised microbial therapies.
Dr Luned Badder (Cardiff University) is an emerging innovator in cancer virotherapy. She is developing a viral platform for “in-tumour chemotherapy”, enabling targeted delivery of therapeutic genes directly to tumour tissue. In her recent publication “The αvβ6 integrin-specific virotherapy, Ad5NULL-A20.FCU1, selectively delivers potent ‘in-tumour’ chemotherapy to pancreatic ductal adenocarcinoma” (https://doi.org/10.1038/s41416-024-02869-3), Dr Badder, Prof. Alan L. Parker, and colleagues developed a novel αvβ6-targeted adenoviral platform capable of delivering suicide genes specifically to αvβ6-positive PDAC cells. The replication-deficient adenovirus encodes a fusion protein of CDase and UPRTase, which directly converts the non-toxic prodrug 5-fluorocytosine (5-FC) into the active chemotherapeutic 5-fluorouracil (5-FU) and its metabolites within tumour tissue. This “in-tumour chemotherapy” approach minimises systemic toxicity while maintaining potent anti-cancer efficacy. In another study, “An αvβ6-specific virotherapy expressing bispecific immune cell activators induces immune cell activation to mediate tumour cell death” (https://doi.org/10.1016/j.omton.2025.201017), Dr Badder and colleagues demonstrated that the αvβ6-targeted adenoviral platform can deliver bispecific immune cell activators (BICAs), driving T cell and NK cell infiltration into tumour organoids and promoting potent, tumour-specific cell killing.
Together, Dr Korcsmáros and Dr Badder’s work underscores a powerful union: organoids as physiological mini-tissues, cytokine and viral perturbations as tools and stressors, and computational insights to map complex biological responses.
WORC.Community would like to thank its event commercial partners: QKine, LICORbio, Bio-techne & Network partner Crown Bioscience


Please sign in or register for FREE
If you are a registered user on WORC.Community, please sign in